邀请科研好友扫码注册
易科学送惊喜!
邀请码:



恭喜您获得
“易科学200元科研券”
![]() 姜老师
姜老师
-

首页
-
严选测试
-

消息
0 -
提交需求
-

我的
服务简介
服务详情
详细说明
1 Test Method
1.1 Treatment Group
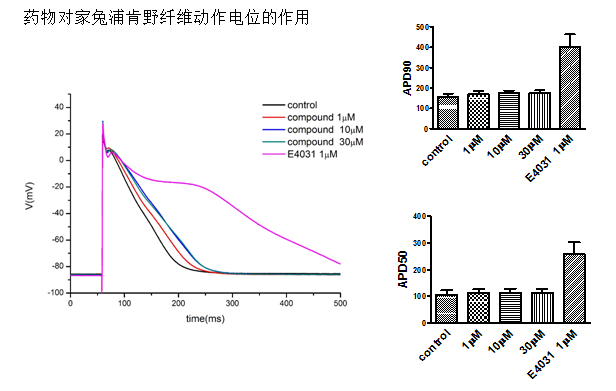
Three (3) concentrations of the Test Article be applied sequentially in ascending concentration to each Purkinje fiber. Each concentration will be tested in at least four (4) fibers (n ³ 4).
The test article Vehicle Control will be applied to at least four (4) fibers (n ³ 4) with exposure times to approximate those of the test group. At the end of the vehicle exposure period the Positive Control article (sotalol at 100 µM) will be applied.
1.2 Heart preparation
Rabbits will be purchased from Xiehe Jianhao (Beijing) in this study. Heparin will be given (500 U/kg i.v.) followed by sodium pentothal (20-40 mg/kg, i.v.). The skin of the entire chest area will be shaved. After anesthesia is confirmed, the heart will be removed rapidly through a left lateral thoracotomy. The heart will be immediately placed into cold, oxygenated cardioplegic Tyrodes solution (modified to contain 26 mM KCl). The heart will be placed in a large glass beaker and flushed with Tyrode’s solution to remove blood.
1.3 Electrophysiological Procedures
A Purkinje fiber will be placed in a recording chamber mounted on a heated platform, and superfused at approximately 2 mL /min with vehicle control solution. The bath temperature will be controlled using a water bath chamber. Bath temperature will be measured using a thermistor probe. Intracellular membrane potentials will be recorded using conventional intracellular microelectrodes pulled from borosilicate glass capillary tubing on a micropipette puller (Sutter Instruments P-97, Novato, CA) filled with 3 M KCl solution.
Action potentials will be evoked by repetitive electrical stimuli (1-3 ms duration, approximately 1.5 times threshold amplitude). A bipolar, insulated (except at the tip) electrode will be used to deliver pulses generated by a photo-isolated, electronic stimulator. Analog signals will be low-pass filtered at 10 kHz before digitization at 50 kHz, and stored on hard disk using a PC-compatible computer controlled by Axon Pclamp 10.2 software.
1.4 Test Procedures
Concentration-response and rate-dependence will be determined by the following test procedure (summarized in the schedule table below). Purkinje fibers will be paced continuously at a basic cycle length (BCL) for at least 30 minutes (recovery and stabilization) before obtaining control AP responses. Only fibers with resting potentials more negative than –80 mV and normal AP morphology will be used. After recovery and stabilization, the control solution will be applied for at least 5 minutes for equilibration in the absence of test article and the fiber will be pulsed at a basic cycle length of 2 s. At the end of this period, baseline control APD rate-dependence will be measured using stimulus pulse trains consisting of approximately 50 pulses at BCL of 2, 1 and 0.5 s. For test group, after returning to BCL of 2 s, test article at the lowest concentration will be applied for at least 20 minutes to allow equilibration, and the stimulus trains repeated. The entire sequence (~20 minutes of equilibration followed by stimulus trains at decreasing BCL) will be repeated at cumulatively increased test article concentration. For vehicle control group, entire sequence will be repeated with time-matched vehicle controls. The sequence for the positive control will be repeated at end of vehicle controls. The average responses from the last five recorded action potentials from each stimulus train will be analyzed for each test condition.
Stimulus and Solution Application Schedule
Solution | Approximate Duration (min) | Measurement | Stimulus Interval, BCL (s) |
Vehicle Control Solution | ³ 30 | Recovery and Stabilization | 0.5 - 2 |
Vehicle Control Solution | ³ 5 | APD rate dependence | 2 |
Vehicle Control Solution | ~ 3 | APD rate dependence | 2, 1, 0.5 |
Test article concentration or Vehicle Control (1·· X) | ³ 20 | Equilibration | 2 |
Test article concentration or Vehicle Control (1·· X) | ~ 3 | APD rate dependence | 2, 1, 0.5 |
100 µM Sotalola | ³ 20 | Equilibration | 2 |
100 µM Sotalola | ~ 3 | APD rate dependence | 2, 1, 0.5 |
a Following the final vehicle group only
(1·· X): Repeated for each test article concentration; X = number of test article concentration to be applied and tested on each fiber.
1.5 Electrophysiological Data Analysis
1.5.1 Action Potential Analysis
Data will be analyzed with Pclamp 10.2 software. The following parameters will be determined: RMP (resting membrane potential, mV), APA (action potential amplitude, mV), Vmax (maximum rate of rise V/s), APD60 and APD90 (action potential duration at 60 and 90% repolarization, respectively, ms). Concentration-response data will be presented relative to baseline before test article application. APD60, APD90 and Vmax at each stimulus frequency will be presented as percent change (D%) from baseline at each concentration. RMP and APA data will be presented as change in membrane potential (DmV).
1.5.2 Statistical Analysis
Data will be reported as Mean ± SEM. Pooled data will be tabulated for each condition: control baseline, test article concentration and stimulus frequency. Changes in action potential parameters will be evaluated using one-way ANOVA followed by Dunnett’s multiple comparison test to determine whether the change from baseline observed after equilibration in each test article concentration is significantly different (P<0.05) from that observed in the time-matched vehicle control group.